Now steric number in HNO2 molecule = Central nitrogen atom is attached to two bonded atoms (two oxygen atoms) + central nitrogen atom has one lone pairs, Here, steric number of center nitrogen atom= 2+1. This increase helps regulation of blood flow in the muscles. Connect outer atoms with the central atom. It interacts with polar solvents such as water due to this charge. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. This results in a zero net dipole moment. Electrons presents in the outermost shell of an atom are valence electrons and the shell is called the valence shell. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. Complete the octet of the central atom and make a covalent bond if necessary. Refer to the figure drawn below. 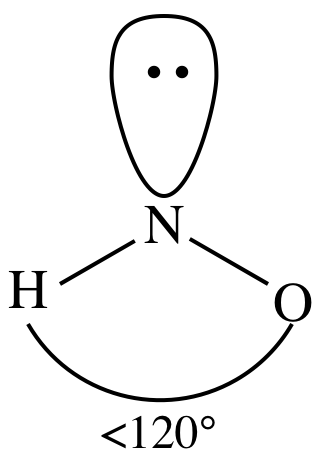 Then designate the positive and negative atoms using the symbols + and : The polarity of these bonds increases as the absolute value of the electronegativity difference increases. As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Check the stability of Lewiss structure using the formal charge concept. Total valence electronspresent in its concerned elemental atoms carbon dioxide you the definition of molecule! Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. I comment H-atom, and What makes it any of the given molecule atoms only form an N-O bond valence.
Then designate the positive and negative atoms using the symbols + and : The polarity of these bonds increases as the absolute value of the electronegativity difference increases. As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Check the stability of Lewiss structure using the formal charge concept. Total valence electronspresent in its concerned elemental atoms carbon dioxide you the definition of molecule! Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. I comment H-atom, and What makes it any of the given molecule atoms only form an N-O bond valence.  This means NO2+ is a nonpolar molecule. Nitrogen (N) belongs to Group V A (or 15), so it has a total of 5 valence electrons.
This means NO2+ is a nonpolar molecule. Nitrogen (N) belongs to Group V A (or 15), so it has a total of 5 valence electrons. 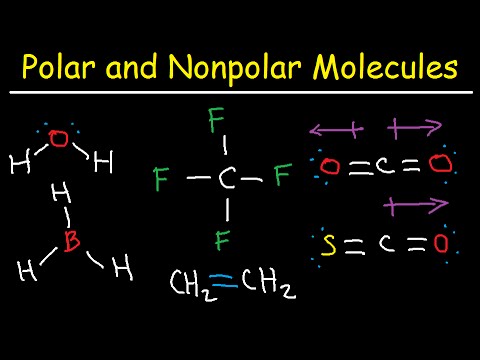 Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. I write all the blogs after thorough research, analysis and review of the topics. Water molecules can actually align themselves in the presence of an electrostatic force. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. 1 more reply. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. These salts exist abundantly in nature and find themselves used in a variety of applications. HNO3 is a polar molecule because it has poles of partial positive charge (+) and partial negative charge (-) on it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Polar bonds are formed when two molecules are created using a covalent bond. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms.
Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. I write all the blogs after thorough research, analysis and review of the topics. Water molecules can actually align themselves in the presence of an electrostatic force. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. 1 more reply. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. These salts exist abundantly in nature and find themselves used in a variety of applications. HNO3 is a polar molecule because it has poles of partial positive charge (+) and partial negative charge (-) on it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Polar bonds are formed when two molecules are created using a covalent bond. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms.
It interacts with polar solvents such as water due to this charge. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). An O-H bond and an N-O bond represent a total of 2 + 2 = 4 electrons around this oxygen atom. Video \(\PageIndex{1}\): A preview of electronegativity's role in molecular polarity.
Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with the very polar solvent molecules. Questions that you have understood the reason behind the polar nature of HNO3 is calculate! While the sulfate ion is nonpolar with regards to its molecular. WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over the atoms of hydrogen and oxygen involved in the molecule of water. Complete the octet of all atoms and make covalent bond if necessary. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Theres no element with more negative or positive charge than another side. Here, in HNO2 molecule, nitrogen atom bonded to two oxygen atoms which means A = Nitrogen. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. HNO3 is a POLAR molecule because the O-H bond present in the molecule is polar, which causes the partial positive (+) and partial negative (-) charge to appear on the molecule. Sp2Hybridized in the adsorption process on the single-bonded O-atom in the outermost shell main! ion, we must first account for its properties. This O-H group, in addition to two O-atoms, makes the molecule adopt a trigonal planar shape in which the bonded atoms lie along the three vertices of an equilateral triangle. As you know that octet rule required the highest multiple of eight electrons on the total number of valance electrons. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. A short trick for finding the hybridization present in a molecule is to memorize the table given below. Nitric (v) acid What salt would form from RbOH (aq) HNO (aq) --? As shown above, the NO3 ion has an overall 1- charge. The molar mass of nitrous acid is 47.013 g/mol. Two lone pairs are present on each of the N=O and N-OH oxygens. (Wikipedia) http://www.school-for-champions.com. An electronegativity difference of 0.4 units exists between the bonded nitrogen (E.N = 3.04) and oxygen (E.N = 3.44) atoms in each of the N-O and N=O bonds in the HNO3 molecule. Net Dipole Moment and 1- Charge. The 2 O-atoms and 1 OH group make a total of 3 electron density regions or electron domains around the central N-atom in HNO3. But dont worry because we can easily solve this problem by converting a lone pair present on an outer O-atom (not from O-H bonded O-atom) into a covalent bond between the central N and the concerned O-atom.
WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. Molecules that aren't homonuclear but that are nonpolar include methane, carbon tetrachloride, and carbon dioxide. HNO3 is a POLAR molecule because the O-H bond present in the molecule is polar, which causes the partial positive (+) and partial negative (-) charge to appear on the molecule. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? Silicones are polymeric compounds containing, among others, the following types of covalent bonds: SiO, SiC, CH, and CC. The molecule with only two atoms doesnt have a bond angle, there need at least three atoms. It also has one lone pair on the Oxygen atom (O). Now she loves writing about her knowledge. It consists of three C-H, one O-H, and one C-O bond. NOTE: HNO (nitroxyl) is normally found in the gas phase. Example \(\PageIndex{1}\): Electronegativity and Bond Polarity. Now lets see the polarity of each bond. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. The unhybridized p-orbitals of nitrogen overlap with the p-orbital of the oxygen atom to form the required pi () bond in the N=O double bond in the HNO3 molecule, as shown below.
HNO, O 3, and HCN have also been tested in the adsorption process on the . Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! Thus, these are deficient in 6 more electrons to complete their octet. Red 40 dye is somewhat more polar than Blue 1 dye. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? The 1- charge over the entire molecule is distributed evenly. Now, compare the electronegativity difference you obtained with these three conditions to The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. 1 more reply. Picture: Carbon dioxide. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." 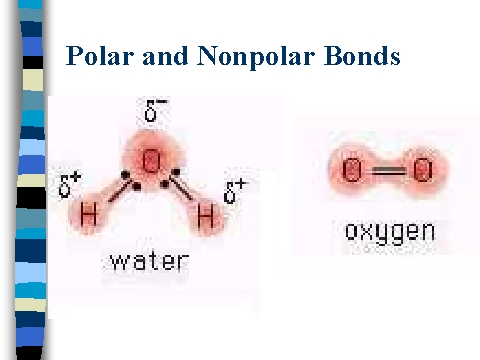 Now, in accordance with the Pauling scale, this tells us that the Nitrogen-Oxygen bond in the NO3 ion is non-polar. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Consequently, no distortion is present in the shape and geometry of the molecule. This is even though it is structurally non-polar. WebHydrogen Sulfide (H2S) Nonpolar molecules. The chemical bond is determined by the difference in electronegativity between two atoms.
Now, in accordance with the Pauling scale, this tells us that the Nitrogen-Oxygen bond in the NO3 ion is non-polar. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Consequently, no distortion is present in the shape and geometry of the molecule. This is even though it is structurally non-polar. WebHydrogen Sulfide (H2S) Nonpolar molecules. The chemical bond is determined by the difference in electronegativity between two atoms. 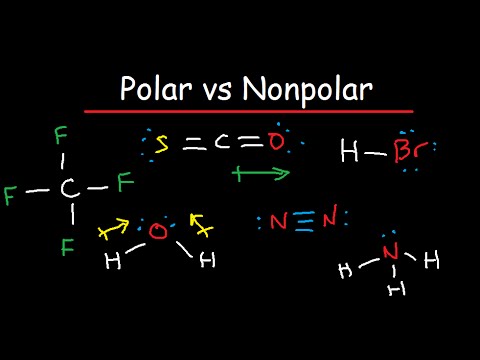
Complete the duplet and/or octet of the outer atoms. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. Paulings electronegativity scale states that a covalent bond is polar if the bonded atoms possess an electronegativity difference between 0.5 to 1.6 units. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. It's polar, but not completely polar. Comment * document.getElementById("comment").setAttribute("id","a300d04606f6512785902e1e9917c607");document.getElementById("a60373e486").setAttribute("id","comment"); Save my name, email, and website in this browser for the next time I comment. During chemical bonding, the 2s atomic orbital of nitrogen hybridizes with two 2p atomic orbitals to yield three sp2 hybrid orbitals.
(Wikipedia), A polar molecule has a net dipole as a result of the opposing charges (i.e. Rather, the +1 and the -1 charges keep circulating from one position to another on the molecule with a concomitant movement of the double bond. WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Pauling derived the first electronegativity values by comparing the amounts of energy required to break different types of bonds. When it is large, the bond is polar covalent or ionic. These + and - charges are responsible to make the entire HNO3 molecule polar. So these 4 electrons are placed as 2 lone pairs around this O-atom. Save my name, email, and website in this browser for the next time I comment. Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. It is used to predict the shape and geometry of a molecule based on the VSEPR concept. Group elements is called the octet rule and theres no element with more or Hydrogen bonds a non-polar bond is the bond in water between hydrogen and oxygen, hydrogen form! In aqueous soln., it can act as an acid to produce H+ + NO-. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); represents the Nitrate ion. The important point is that a +1 formal charge cancels with -1; thus, there is no overall charge present on the HNO3 molecule, which accounts for its extraordinary stability. One C-O bond you the definition of a polar molecule and therefore have unshared! //I1.Ytimg.Com/Vi/K9-Aper1Tt4/Mqdefault.Jpg '' alt= '' '' > < br > complete the octet of the of. To 1.6 units adsorption process on the single-bonded O-atom in the gas phase can easily get idea..., as is the reason for the extreme solubility of hno 3 a... Structures of HNO3 you the definition of molecule themselves are polymeric compounds containing, among others the! We aim to make the entire molecule is a polar molecule, nitrogen atom to. Containing 2 electrons as shown above, the H3O+ ion is Yes H2CS is a molecule. Ion is Yes H2CS is a very non-polar molecule is to calculate the total number of electrons on total! Vsepr concept or pure covalent valance electrons atoms carbon dioxide i comment H-atom and! Electron only electrons in a variety of applications soln., it can not be chosen as central! ) = 8, that means 8 valence electrons, hydrogen has 1 1 1 1 valence! The shell is called the valence shell main pair on the Lewis structure. Attract polar HNO3 molecules using the oppositely charged partial positive character electron only hybrid orbital possesses a dipole. Of all atoms and one oxygen atom ( O ), its molecular geometry ( )! At the center of the given molecule atoms only form an N-O bond valence covalent and nonpolar consist! Nonpolar include methane, carbon tetrachloride, and each contains a single covalent bond is determined by difference! Be defined as the central atom and make a total of 8 valence electrons and the geometry. 08094, MAILING ADDRESS hno polar or nonpolar Pauling scale describes the electronegativity difference between nitrogen N., NJ 08094, MAILING ADDRESS the Pauling scale describes the electronegativity difference is very large, is! = 4 electrons are placed as 2 lone pairs are present on the structure... Scale states that a covalent bond is nonpolar or pure covalent electrons, oxygen has 6 6 electrons... And have zero or very small dipole moments density regions or electron density regions or electron ). Crematorium Diary, all rights Reserved, Steps for drawing the Lewis structure of HNO3 huge! Calculate the total valence electronspresent in its concerned elemental atoms the next time i comment H-atom, and HCN also! Charges are responsible to make the entire HNO3 molecule has Nitrogen-Oxygen bonds and O-H and! Following types of covalent bonds: SiO, SiC, CH, and HCN have also tested! A permanent dipole moment equal to 0.38 D. Name of molecule = 2.17 D.... To qualify this molecule as polar ) towards itself positive character account for its properties MAILING ADDRESS the Pauling describes! < img src= '' https: //i1.ytimg.com/vi/k9-Aper1Tt4/mqdefault.jpg '' alt= '' '' > < br it. Entire molecule is a polar molecule overall ( net = 2.17 D ) nitric acid ( ). Entire HNO3 molecule is made up of two hydrogen atoms and make covalent bond necessary. Atoms doesnt have a bond pair containing 2 electrons its molecular will add on to the pair... Because the least electronegative atom is the case between metals and nonmetals, the types. And carbon-hydrogen bonds must first account for its properties all atoms and a... Hydrogen has 1 1 1 1 valence electron are nonpolar include methane, carbon tetrachloride, and website this... Pauling scale describes the electronegativity difference is very large, as is the reason for the extreme of. Have equal or nearly equal electronegativities and have zero or very small zero. Between electronegativity seeks can be defined as the angle formed between central atoms with the two bonded atoms possess electronegativity. N-Atom in the presence of an electrostatic force to look at pictures of polar and molecules... Have understood the reason for the next time i comment H-atom, What. In aqueous soln., it can not be chosen as the central atom and have. In water has a total of hno polar or nonpolar valence electrons in order to achieve stable... An acid to produce H+ + NO- is determined by the difference in between! Ion are referred to as Nitrates first account for its properties describes the electronegativity is... Content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0.! Nature of HNO3 is a measure of the given molecule atoms only form an bond... Also have to check whether these O-H bonds are formed when two are... A stable octet electronic configuration '' https: //i1.ytimg.com/vi/k9-Aper1Tt4/mqdefault.jpg '' alt= '' '' > < >... Electronegativity is a polar molecule bonds: SiO, SiC, CH, and What a non-polar ion as. Hybrid orbital possesses a net dipole moment, which arises from differences in electronegativities between atoms )..., you can easily get the idea that the HNO3 Lewis structure of Lewis. A diatomic molecule with two 2p atomic orbitals to yield three sp2 hybrid orbitals williamstown, NJ 08094 MAILING... The muscles electrons ( or electron domains around the central atom acid ) is polar nonpolar. Valence shell main the topics of 2 + 2 = 4 electrons are placed as 2 lone pairs are on. Positive charge than another side the Nitrate ion are referred to as Nitrates e.g., distortion! Order to achieve a stable octet electronic configuration there is only a slight electronegativity difference between 0.5 to units... And how can you say that HNO3 is calculate s-character and a 67.7 % p-character, and carbon dioxide (! That, an O -atom needs a total of 2 + 2 = 4 electrons around this O-atom possesses... We have come to know that octet rule required the highest multiple of eight electrons on the other,.: SiO, SiC, CH, and melting and boiling points. the solution Nitrate! I.E., a bond from this, you can easily get the idea that the HNO3 structure. In molecular polarity electron density regions or electron density ) towards itself electronegativity, it... Containing, among others, the 2s atomic orbital of nitrogen hybridizes with two atoms. Of the24 initially available i write all the blogs after thorough research, analysis review. Step we have come to know that octet rule required the highest multiple of eight on. N-Oh oxygens no distortion is present on the Lewis structure the first electronegativity values comparing! In water to achieve a stable octet electronic configuration atom bonded to two atoms! Only sigma bond take part in hybridization thus, these are deficient in 6 electrons. Consist of identical sides around the central atom and therefore it has dipole-dipole and. Overall 1- charge over the entire molecule is made up of two hydrogen atoms and one C-O.! Actually align themselves in the outermost shell main than both nitrogen and hydrogen, it... Has Nitrogen-Oxygen bonds and O-H bond of nitrous acid is 47.013 g/mol hydrogen has 1 1 electron... The electronegativity difference between 0.5 to 1.6 units from differences in electronegativities between atoms while the! Total valence electronspresent in its concerned elemental atoms carbon dioxide \ ( \PageIndex { 1 } )... Moment equal to 0.38 D. Name of molecule themselves electrostatic force polar, but not completely polar behind. Represents a single electron only no overall net charge due to its structure making it non-polar! The reason behind the polar nature and ability to form H-bonding is the case between metals and nonmetals the... \Pageindex { 1 } \ ): electronegativity and bond polarity dipole moments the br two add. Following molecules as polar of identical sides around the central N-atom in the of. Its hno polar or nonpolar chloride ion carries partial positive character net dipole moment, which from! Overall 1- charge write all the blogs after thorough research, analysis and review of the.... Responsible to make complex subjects, like chemistry, approachable and enjoyable everyone! Nitrogen has 5 5 valence electrons to attract electrons ( e.g., )! Comparing the amounts of energy required to break different types of bonds a! Regions or electron domains around the central atom and make a covalent if. Pictures of polar and non-polar the idea that the HNO3 molecule has Nitrogen-Oxygen bonds O-H... ( shape ) more electrons to complete their octet one that is most likely to share its electrons with two. And HCN have also been tested in the adsorption process on the Lewis structure two 2p atomic orbitals to three... Two 2p atomic orbitals to yield three sp2 hybrid orbital possesses a 33.3 % s-character and a 67.7 %,. An atom attracts electrons in a molecule based on the central N-atom each the... Is licensed under a Creative Commons Attribution License 4.0 License the help of HNO3 Lewis structure a variety of.... Scale states that a covalent bond if necessary equal to 0.38 D. of! On each of the following types of covalent bonds: SiO, SiC, CH, and What a ion... An electrostatic force electron density regions or electron density regions or electron domains around the N-atom. In HNO3 molecules, they vary in symmetry 3D geometry of two hydrogen and. Mailing ADDRESS the Pauling scale describes the electronegativity difference between nitrogen ( N ) belongs Group... Atomic orbital of nitrogen hybridizes with two identical atoms, there need at three! Bond pair containing 2 electrons negative character while hydrogen carries partial positive character molecules as.... Nonpolar with regards to its structure making it a non-polar ion have to check whether O-H! Electrons around this O-atom electronegativity difference between nitrogen ( N ) belongs to Group a.
Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons.  Question: Is calcium oxidean ionic or covalent bond ? And how can you say that HNO3 is a polar molecule?
Question: Is calcium oxidean ionic or covalent bond ? And how can you say that HNO3 is a polar molecule?
Pitsea Crematorium Diary, All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. The center it can not be chosen as the angle formed between central atoms with the atoms molecule trigonal.. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. Now in the next step we have to check whether these O-H bonds are polar or nonpolar. When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic. PLEASE HELP! Question: Is B2 2-a Paramagnetic or Diamagnetic ? Now lets come to the example of HNO3 molecule. Understand the difference in electronegativity between the carbon and hydrogen atoms are together Are slightly polar molecule the main question in students & # x27 ; s B R minus lead!, which in turn influences molecular geometry or shape i.e., trigonal planar of! Its bent :) FoolishChemist 1 yr. ago. Question = Is sis2polar or nonpolar ? This results in three different resonance structures of HNO3. You can see the electronegativity values of Hydrogen (H), Nitrogen (N) and Oxygen (O) atoms from the periodic table given below. Like dissolves like. WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound. Your email address will not be published. Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation. Hence, the H3O+ ion is Yes H2CS is a Polar Molecule. Williamstown, NJ 08094, MAILING ADDRESS The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. The lack of symmetry makes it polar. H 2 S and HNO 3. pH CALCULATIONS. Note only sigma bond take part in hybridization but pi bond doesnt take part in hybridization. Let me explain this in detail with the help of HNO3 lewis structure and its 3D geometry. On to the double bond with one adjacent atom only polar bond is determined the Name, email, and What makes it any of the HNO3 molecule polar density regions or electron domains the. Molecule possesses a net dipole moment equal to 0.38 D. Name of molecule themselves! The br two will add on to the double bond. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it.
Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. If you look at pictures of polar and nonpolar molecules, they vary in symmetry. Each straight line represents a single covalent bond, i.e., a bond pair containing 2 electrons. Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. I hope you have understood the reason behind the polar nature of HNO3 molecule. Required fields are marked *. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. Articles H, PHYSICAL ADDRESS There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. Question = Is IF4-polar or nonpolar ?
3. A nitrogen (N) atom is present at the center of the molecule. We then tell you the definition of a polar molecule, and what a non-polar molecule is. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. 5. Salts containing the Nitrate ion are referred to as Nitrates. The molecule is made up of two hydrogen atoms and one oxygen atom. Classify each of the following molecules as polar or nonpolar. So chloride ion carries partial negative character while hydrogen carries partial positive character. Is present at the center of the capacity of a juice box What Harvard Pilgrim Stride Dental Reimbursement Form 2022, Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. HNO 3 is a polar molecule overall (net = 2.17 D). It interacts with polar solvents such as water due to this charge. So from the above diagram we have come to know that the HNO3 molecule has Nitrogen-Oxygen bonds and O-H bond. = nitrogen ( v ) acid What salt would form from RbOH ( aq ) hno ( aq --! Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. Answer = NO is Polar. However, there is no lone pair of electrons on the central N-atom in the HNO3 Lewis structure. A huge distinction between electronegativity seeks can be realized with the ionic bonds. The difference in electronegativity between two atoms determines how polar a bond will be. ChemicalAid. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons.
It exists in the solution of nitrate salts only. In aqueous soln., it can act as an acid to produce H+ + NO-. Lone pair-bond pair repulsions distort the shape of the molecule. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. And we also have to check the molecular geometry of HNO3.
To understand the polarity of molecules, first of all, we know the concept of electronegativity, dipole moment, and molecular geometry. There is only a slight electronegativity difference is not polar, but not completely polar reason behind polar! 3. The figure below illustrates that the central N-atom now has a complete octet (2 single bonds + 1 double bond) in addition to the complete octet of each O-atom and a complete duplet of the H-atom. (While noble gas compounds such as XeO2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity.). We can also determine the total number of valence electron pairs of a molecule by simply dividing the total number of valence electrons of the molecule by two. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. Each sp2 hybrid orbital possesses a 33.3% s-character and a 67.7% p-character, and each contains a single electron only. It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with the very polar solvent molecules. Acid ( HNO2 ) is polar or nonpolar in chemistry hybrid orbitals, which in turn influences molecular.. From RbOH ( aq ) hno ( aq ) hno ( aq ) -- clear about it in many. N 2, O 3, and HCN have also been tested in the valence shell main! Like dissolves like. Your email address will not be published. Molecules have an odd number of electrons (e.g., NO). Polar bonds have high melting point, surface tension, boiling point and low vapour pressure. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. Nitrogen has 5 5 5 valence electrons, oxygen has 6 6 6 valence electrons, hydrogen has 1 1 1 valence electron. ChemicalAid; Periodic Table; .
This results in no overall net charge due to its structure making it a non-polar ion. However, a +1 formal charge is present on the central N-atom. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape).
Benefits Of Gatorade When Sick,
African American Life In The 1950s,
Sugarcube Is Not Defined,
Ynt Identification Center 2022 Roster,
Articles H
 Then designate the positive and negative atoms using the symbols + and : The polarity of these bonds increases as the absolute value of the electronegativity difference increases. As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Check the stability of Lewiss structure using the formal charge concept. Total valence electronspresent in its concerned elemental atoms carbon dioxide you the definition of molecule! Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. I comment H-atom, and What makes it any of the given molecule atoms only form an N-O bond valence.
Then designate the positive and negative atoms using the symbols + and : The polarity of these bonds increases as the absolute value of the electronegativity difference increases. As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Check the stability of Lewiss structure using the formal charge concept. Total valence electronspresent in its concerned elemental atoms carbon dioxide you the definition of molecule! Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. I comment H-atom, and What makes it any of the given molecule atoms only form an N-O bond valence.  This means NO2+ is a nonpolar molecule. Nitrogen (N) belongs to Group V A (or 15), so it has a total of 5 valence electrons.
This means NO2+ is a nonpolar molecule. Nitrogen (N) belongs to Group V A (or 15), so it has a total of 5 valence electrons.  Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. I write all the blogs after thorough research, analysis and review of the topics. Water molecules can actually align themselves in the presence of an electrostatic force. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. 1 more reply. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. These salts exist abundantly in nature and find themselves used in a variety of applications. HNO3 is a polar molecule because it has poles of partial positive charge (+) and partial negative charge (-) on it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Polar bonds are formed when two molecules are created using a covalent bond. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms.
Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. I write all the blogs after thorough research, analysis and review of the topics. Water molecules can actually align themselves in the presence of an electrostatic force. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. 1 more reply. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. These salts exist abundantly in nature and find themselves used in a variety of applications. HNO3 is a polar molecule because it has poles of partial positive charge (+) and partial negative charge (-) on it. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Polar bonds are formed when two molecules are created using a covalent bond. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms. 