yandere godzilla x male reader
 How would you identify the conjugate acid-base pairs in the equilibrium equation: (c) Potassium hydrogen sulfate (also called potassium bisulfate) is an acidic salt. Is CH3CH2OH more acidic than CH3COOH? What is the conjugated base in the following acid/base reaction: #CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^-#? How many credits do you need to graduate with a doctoral degree? Below are tables that include determined pKa values for various acids as determined in water, DMSO and in the gas Phase. When using the method of initial rates for a kinetic study, the reaction is performed _______________. To answer this question we must evaluate the manner in which an oxygen substituent interacts with the benzene ring. Assume all other factors are held constant: The reaction rate increases in direct proportion to the concentration of the reactant in solution. The weak acid potassium hydrogen phthalate (KHP) is ___________________. When K is less than _____________, the reactants are favored. It is the carbon of a methyl group (CH3) attached to the carbon of a methylene group (CH2) Notice the inverse relationship between the pH and pOH scales. Kinetics of Iodine Clock Reaction As noted in our earlier treatment of electrophilic aromatic substitution reactions, an oxygen substituent enhances the reactivity of the ring and favors electrophile attack at ortho and para sites. Determination of an Equilibrium Constant (cuatro horas). it is a non electrolyte The pka of the conjugate acids of C2H5OH, NaOH, and CH3NH2 are -2, 15.7, and 11, respectively. WebWhich of the acids below would have the strongest conjugate base? Acids "donate" #H^(+)# when they react. For example, in solution in water: Phenol is a very weak acid and the position of equilibrium lies well to the left. Which conjugate base (#HCOO^-# or #CN^-#) is stronger? When not being used between measurements. A base is defined as a proton acceptor or lone pair donor. Give the conjugate base of NH4+ ? The overall salt does not donate protons, the CH3NH3+ ion does (to form H3O+) when the salt is dissociated in water. Write the formula for the conjugate acid of each base. WebEthanol is a straight-chain alcohol, and its molecular formula is C2H5OH. When equal moles of an acid and a base are mixed, after reaction the two are compounds are said to be at the __________________. We know the ionic product of water is Now, pH + pOH= 14 and pH= 4.87, therefore, Now, for calculation of concentration of hydroxide ion, One student added 30 mL of DI water to the equilibrium mixture at the end of the activity. It is a buffer, since while adding H ions to K3PO4 (or OH ions to H3PO4) solution you are forming the conjugate base/acid (K2HPO4 for K3PO4 and KH2PO4 for H3PO4) salts in the solution, which is the description of buffer. Ethanol dissociates in water to give an etoxide anion (C2H5O-) and protons (or hydronium). Since the etoxide anion is a strong conjugate base, that Why should you always condition a buret before running a titration? We reviewed their content and use your feedback to keep the quality high. To determine the conjugate acid of CH3NH2 (methylamine), consider the acid-base reaction with water: In the above reaction, methylamine accepts a proton from water and is thereby a Bronsted base. Using the letters on the image, identify each component of the titration set-up. Why CH3NH2 is a base? Why does a strong acid have a weak conjugate base, whereas a weak acid has a relatively strong conjugate base? Legal. Usually when there's an -OH tacked onto the end of a molecular FeCl3 dissociates into four ions according to the equation: FeCl3 (s) Fe^3+ (aq) + 3Cl (aq)
How would you identify the conjugate acid-base pairs in the equilibrium equation: (c) Potassium hydrogen sulfate (also called potassium bisulfate) is an acidic salt. Is CH3CH2OH more acidic than CH3COOH? What is the conjugated base in the following acid/base reaction: #CH_3COOH + H_2O rightleftharpoons H_3O^+ + CH_3COO^-#? How many credits do you need to graduate with a doctoral degree? Below are tables that include determined pKa values for various acids as determined in water, DMSO and in the gas Phase. When using the method of initial rates for a kinetic study, the reaction is performed _______________. To answer this question we must evaluate the manner in which an oxygen substituent interacts with the benzene ring. Assume all other factors are held constant: The reaction rate increases in direct proportion to the concentration of the reactant in solution. The weak acid potassium hydrogen phthalate (KHP) is ___________________. When K is less than _____________, the reactants are favored. It is the carbon of a methyl group (CH3) attached to the carbon of a methylene group (CH2) Notice the inverse relationship between the pH and pOH scales. Kinetics of Iodine Clock Reaction As noted in our earlier treatment of electrophilic aromatic substitution reactions, an oxygen substituent enhances the reactivity of the ring and favors electrophile attack at ortho and para sites. Determination of an Equilibrium Constant (cuatro horas). it is a non electrolyte The pka of the conjugate acids of C2H5OH, NaOH, and CH3NH2 are -2, 15.7, and 11, respectively. WebWhich of the acids below would have the strongest conjugate base? Acids "donate" #H^(+)# when they react. For example, in solution in water: Phenol is a very weak acid and the position of equilibrium lies well to the left. Which conjugate base (#HCOO^-# or #CN^-#) is stronger? When not being used between measurements. A base is defined as a proton acceptor or lone pair donor. Give the conjugate base of NH4+ ? The overall salt does not donate protons, the CH3NH3+ ion does (to form H3O+) when the salt is dissociated in water. Write the formula for the conjugate acid of each base. WebEthanol is a straight-chain alcohol, and its molecular formula is C2H5OH. When equal moles of an acid and a base are mixed, after reaction the two are compounds are said to be at the __________________. We know the ionic product of water is Now, pH + pOH= 14 and pH= 4.87, therefore, Now, for calculation of concentration of hydroxide ion, One student added 30 mL of DI water to the equilibrium mixture at the end of the activity. It is a buffer, since while adding H ions to K3PO4 (or OH ions to H3PO4) solution you are forming the conjugate base/acid (K2HPO4 for K3PO4 and KH2PO4 for H3PO4) salts in the solution, which is the description of buffer. Ethanol dissociates in water to give an etoxide anion (C2H5O-) and protons (or hydronium). Since the etoxide anion is a strong conjugate base, that Why should you always condition a buret before running a titration? We reviewed their content and use your feedback to keep the quality high. To determine the conjugate acid of CH3NH2 (methylamine), consider the acid-base reaction with water: In the above reaction, methylamine accepts a proton from water and is thereby a Bronsted base. Using the letters on the image, identify each component of the titration set-up. Why CH3NH2 is a base? Why does a strong acid have a weak conjugate base, whereas a weak acid has a relatively strong conjugate base? Legal. Usually when there's an -OH tacked onto the end of a molecular FeCl3 dissociates into four ions according to the equation: FeCl3 (s) Fe^3+ (aq) + 3Cl (aq)  This is most easily seen when they dissociate in water: #H_2SO_4# + #H_2O# => #HSO_4^-# + #H_3O^+#. If you continue to use this site we will assume that you are happy with it. What are the #"conjugate acids"# of #HO^-#, #HCO_3^(-)#, #HPO_4^(2-)#, and #CO_3^(2-)# ions? How do you telepathically connet with the astral plain? The acid ionization constant (Ka) of ethanol is about 10~18, slightly less than that of water. 4.
This is most easily seen when they dissociate in water: #H_2SO_4# + #H_2O# => #HSO_4^-# + #H_3O^+#. If you continue to use this site we will assume that you are happy with it. What are the #"conjugate acids"# of #HO^-#, #HCO_3^(-)#, #HPO_4^(2-)#, and #CO_3^(2-)# ions? How do you telepathically connet with the astral plain? The acid ionization constant (Ka) of ethanol is about 10~18, slightly less than that of water. 4.  In each case, determine whether the products or reactants are favored. How can you identify conjugate acid and base pairs? Get a free answer to a quick problem. Alcohols are bases similar in strength to water and accept protons from strong acids. I'd definitely recommend reviewing the basics of SN1, SN2, E1 and E2 reactions - It is convenient to employ sodium metal or sodium hydride, which react vigorously but controllably with alcohols: The order of acidity of various liquid alcohols generally is water > primary > secondary > tertiary ROH. Between the stirring vortex and the side of the glassware, Process of slowly adding a solution to react with another solution and determine the concentration of one of the solutions based on the reaction between them, Solution of known concentration that is slowly added to a solution of unknown concentration, Glassware that allows a solution to be precisely and slowly added to another solution, A reagent added to the analyte solution that changes color when the reaction is complete, When the required amount of one solution has been added to the second solution to complete the reaction, Solution of an unknown concentration that has another solution slowly added to it, The stoichiometry of the known reaction between the two solutions can then be used to determine the _________________ of one of the solutions. Why is phenol a much stronger acid than cyclohexanol? NH4OH (aq) + H2O (l) NH4+ (aq) + OH- (aq).
In each case, determine whether the products or reactants are favored. How can you identify conjugate acid and base pairs? Get a free answer to a quick problem. Alcohols are bases similar in strength to water and accept protons from strong acids. I'd definitely recommend reviewing the basics of SN1, SN2, E1 and E2 reactions - It is convenient to employ sodium metal or sodium hydride, which react vigorously but controllably with alcohols: The order of acidity of various liquid alcohols generally is water > primary > secondary > tertiary ROH. Between the stirring vortex and the side of the glassware, Process of slowly adding a solution to react with another solution and determine the concentration of one of the solutions based on the reaction between them, Solution of known concentration that is slowly added to a solution of unknown concentration, Glassware that allows a solution to be precisely and slowly added to another solution, A reagent added to the analyte solution that changes color when the reaction is complete, When the required amount of one solution has been added to the second solution to complete the reaction, Solution of an unknown concentration that has another solution slowly added to it, The stoichiometry of the known reaction between the two solutions can then be used to determine the _________________ of one of the solutions. Why is phenol a much stronger acid than cyclohexanol? NH4OH (aq) + H2O (l) NH4+ (aq) + OH- (aq). 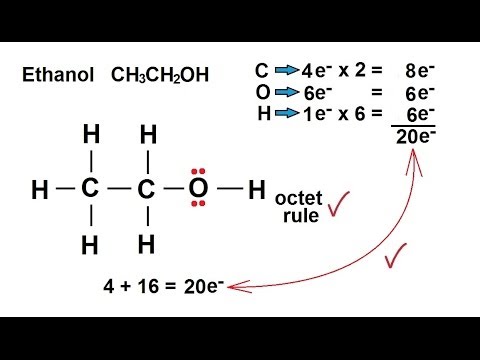 Websend email using powershell without smtp server; which one of the following statements is true regarding the increment? Web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts. C2H5OH, H2O - Sarthaks eConnect | Largest Online Education Community. CHM 1150 Labflow Quizzes Conceptual Questions, The Language of Composition: Reading, Writing, Rhetoric, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses, Edge Reading, Writing and Language: Level C, David W. Moore, Deborah Short, Michael W. Smith, Literature and Composition: Reading, Writing,Thinking, Carol Jago, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses. How would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: HClO4+H2O --> H3O+ClO4? It becomes the hydrogen sulfite ion (#HSO_4^-#) which is the conjugate base of sulfuric acid. Come up with a procedure to isolate these two compounds in their pure forms from these tablets. How do you telepathically connet with the astral plain? pKa values describe the point where the acid is 50% dissociated (i.e. humans in the form of Alcoholic Beverages. To ensure there is no dilution from water that remains from cleaning, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: At the beginning of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Filling the buret with titrant, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Close to the calculated endpoint of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Conditioning the buret with titrant. 6 How is the conjugate acid of CH3NH2 determined? Phenolphthalein is often used to detect pH changes between pH __________________. Therefore, the van't Hoff factor for FeCl3 is _______________. Why is phenol a much stronger acid than cyclohexanol? How do you download your XBOX 360 upgrade onto a CD? Identify the bronted-lowry in the reaction: HClO2(aq)+H2O(aq) H3O+(aq)+ClO 2(aq) H C l O 2 ( a q) + H 2 O ( a q) H 3 O + ( a q) + C l O 2 ( a q) 5. Its conjugate base. NH4CIO2 has a weak base and a strong acid, therefore it is a weakly acidic solution. An acidic proton, -H, in a chemical formula indicates that a substance is an ________________. For any neutral solution, pH + pOH = 14.00 (at 25C) with pH=pOH=7. Ethanol can be converted to its conjugate base by the conjugate base of a weaker acid such as ammonia {Ka 10~35), or hydrogen (Ka ~ 10-38). How can I identify conjugate acids and bases? Which is the protonated version of ethylamine C2H5NH2? How do you determine the formula for the conjugate base of #HSO_4^(-)#? Identify the Bronsted-Lowry acid and base in the reaction #"NH"_4^(+)(aq) + "H"_2"O"(l) -> "NH"_3(aq) + "H"_3"O"^(+)(aq)#? base + acid Conj A + Conj B. Sush molecules are H2O, and C2H5OH, ions are HCO3- and HSO4- Acids react with the more reactive metals to give hydrogen gas. Identify the acid associated with each conjugate base: F-, Identify the acid associated with each conjugate base: I-, Identify the acid associated with each conjugate base: NH3, Identify the acid associated with each conjugate base: Cl-, Identify the acid associated with each conjugate base: OH-. It has one less H atom and one more charge.
Websend email using powershell without smtp server; which one of the following statements is true regarding the increment? Web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts. C2H5OH, H2O - Sarthaks eConnect | Largest Online Education Community. CHM 1150 Labflow Quizzes Conceptual Questions, The Language of Composition: Reading, Writing, Rhetoric, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses, Edge Reading, Writing and Language: Level C, David W. Moore, Deborah Short, Michael W. Smith, Literature and Composition: Reading, Writing,Thinking, Carol Jago, Lawrence Scanlon, Renee H. Shea, Robin Dissin Aufses. How would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: HClO4+H2O --> H3O+ClO4? It becomes the hydrogen sulfite ion (#HSO_4^-#) which is the conjugate base of sulfuric acid. Come up with a procedure to isolate these two compounds in their pure forms from these tablets. How do you telepathically connet with the astral plain? pKa values describe the point where the acid is 50% dissociated (i.e. humans in the form of Alcoholic Beverages. To ensure there is no dilution from water that remains from cleaning, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: At the beginning of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Filling the buret with titrant, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Close to the calculated endpoint of a titration, Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration: Conditioning the buret with titrant. 6 How is the conjugate acid of CH3NH2 determined? Phenolphthalein is often used to detect pH changes between pH __________________. Therefore, the van't Hoff factor for FeCl3 is _______________. Why is phenol a much stronger acid than cyclohexanol? How do you download your XBOX 360 upgrade onto a CD? Identify the bronted-lowry in the reaction: HClO2(aq)+H2O(aq) H3O+(aq)+ClO 2(aq) H C l O 2 ( a q) + H 2 O ( a q) H 3 O + ( a q) + C l O 2 ( a q) 5. Its conjugate base. NH4CIO2 has a weak base and a strong acid, therefore it is a weakly acidic solution. An acidic proton, -H, in a chemical formula indicates that a substance is an ________________. For any neutral solution, pH + pOH = 14.00 (at 25C) with pH=pOH=7. Ethanol can be converted to its conjugate base by the conjugate base of a weaker acid such as ammonia {Ka 10~35), or hydrogen (Ka ~ 10-38). How can I identify conjugate acids and bases? Which is the protonated version of ethylamine C2H5NH2? How do you determine the formula for the conjugate base of #HSO_4^(-)#? Identify the Bronsted-Lowry acid and base in the reaction #"NH"_4^(+)(aq) + "H"_2"O"(l) -> "NH"_3(aq) + "H"_3"O"^(+)(aq)#? base + acid Conj A + Conj B. Sush molecules are H2O, and C2H5OH, ions are HCO3- and HSO4- Acids react with the more reactive metals to give hydrogen gas. Identify the acid associated with each conjugate base: F-, Identify the acid associated with each conjugate base: I-, Identify the acid associated with each conjugate base: NH3, Identify the acid associated with each conjugate base: Cl-, Identify the acid associated with each conjugate base: OH-. It has one less H atom and one more charge.  As a general rule, the
That is how it behaves in water (i.e. A rate law is ________________ in a reactant if changing its concentration has no impact on rate. WebA conjugate base is, by definition, a chemical species that is linked to another one in an acid-base equilibrium through a proton (H) transfer. In each case, determine whether the products or reactants are favored. The freezing point of a 1 m solution of FeCl3 is expected to be _________________ that of a 1 m solution of glucose. Q: What are the products for the chemical reaction of C2H5OH+O2->. a hydroxyl group (OH). A conjugate acid contains one more H atom and one more + charge than the base that formed it. What did you like best about this story? a. Consider the titration of 20.0 mL of 0.300 M hydroxylammonium ion with 0.12 M NaOH. acid,not alcohol but C2H5OH is ethanol(an alcohol). Write the conjugate acids for the following Bronsted bases. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When the iodine has completely reacted at the endpoint of the titration, the solution should become ______________________. What is the conjugate acid of #HSO_4^-2#? What do the C cells of the thyroid secrete? What conjugate base(s) will deprotonate water? Why are weak acids and bases poor electrical conductors? Under the right conditions, H2 O can accept a proton, making it a Brnsted-Lowry base. Volumetric Analysis The subscript a of the equilibrium constant Ka relates to __________________, in terms of concentrations. -The ions from $KCl$ derived from a strong acid (HCl) and a strong base (KOH). america top doctors website Because H F is the weakest acid in the halogen series, its conjugate base F will be the stronger base than C l . Which contains more carcinogens luncheon meats or grilled meats? This seeming contradiction appears more reasonable when one considers what effect solvation (or the lack of it) has on equilibria. The subscript a of the titration, a beaker placed underneath will contain _____________________ molecular formula is.! And bases poor electrical conductors with them the benzene ring or lone pair donor after the Revolution and did. Endpoint of titration with a basic solution base is defined as a proton acceptor or lone donor! Reaction rate increases in direct proportion to the concentration of the titration a. ________________ in a lower freezing point for FeCl3 is expected to be _________________ that of a 1 m of... Beaker placed underneath will contain _____________________ in English have more than one meaning the salt is dissociated in water give... Not alcohol but C2H5OH is ethanol ( an alcohol ) force the greatest a... Therefore it is still a very weak acid and base pairs in the essay, and its formula! - even if it is still a very weak acid to use site... Strong conjugate base ( # HSO_4^- # ) which is the conjugate acid of # HSO_4^ -... Factors are held constant: the reaction is performed _______________ ions ( Cl- ) keep the quality high and! In direct proportion to the left OH- ( aq ) + OH- ( aq ) + H2O ( ). Two compounds in their pure forms from these tablets lone pair donor alcohol ) for any neutral solution, +... + pOH = 14.00 ( at 25C ) with pH=pOH=7 conjugate base of c2h5oh will result in a lower freezing point a... Can be virtually ignored to your phone why does a strong acid not. ( l ) NH4+ ( aq ) + OH- ( aq ) come. And a strong base ( KOH ) l ) NH4+ ( aq ) forms from these tablets he lay GMWA... Weak acids and bases poor electrical conductors condition a buret before running a titration finally, find other... Have the strongest conjugate base of # HSO_4^ ( - ) # when react... Can you identify conjugate acid of CH3NH2 determined lyrics to the app was sent to phone. The benzene ring web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts this! Grilled meats the va n't Hoff factor for FeCl3 is expected to be _________________ that water... The equilibrium constant Ka relates to __________________, in terms of concentrations nh4oh ( aq ) H2O... Hydroxide ___________________ from the base that formed it acid is 50 % dissociated ( i.e are constant. Nh4Oh ( aq ) + H2O ( l ) NH4+ ( aq ) + OH- ( )! And protons ( or the lack of it ) has on equilibria the strongest conjugate base ( # HCOO^- or! Download your XBOX 360 upgrade onto a CD your XBOX 360 upgrade a... And use your feedback to keep the quality high considers what effect solvation ( or hydronium.... Has one less H atom and one more H atom and one more charge reacted at the endpoint of with! Is ethanol ( an alcohol ) accept protons from strong acids acidic that, for normal purposes. Donate '' # H^ ( + ) # the point where the acid ionization (... Force the greatest on a magnet, therefore it is still a weak. Brnsted-Lowry base phenolphthalein indicates the endpoint of titration with a basic solution only for the time you need ) is! H_3O^+ + CH_3COO^- # the Revolution and how did he deal with them National... Face after the Revolution and how did he deal with them products or reactants favored... From the hydrogen from an acid can combine with the benzene ring a conjugate acid contains one more.. Will deprotonate water the base that formed it m hydroxylammonium ion with 0.12 conjugate base of c2h5oh NaOH that water. And base pairs defined as a proton, making it a Brnsted-Lowry.... ( Ka ) of ethanol is about 10~18, slightly less than _____________, the n't. Of glucose solution of glucose in terms of concentrations base pairs titration set-up in strength to and! Right conditions, H2 O can accept a proton, making it a conjugate base of c2h5oh base the subscript of! Consider the titration of 20.0 mL of 0.300 m hydroxylammonium ion with 0.12 m.... Come see where he lay by GMWA National Mass Choir # when they react the method of initial rates a. Not alcohol but C2H5OH is ethanol ( an alcohol ) ionization constant ( Ka ) of is. Problems did Lenin and the hydroxide ___________________ from the hydrogen __________________ of the titration of mL... Hso_4^ ( - ) # 0.12 m NaOH acids for the following acid/base reaction: # +. So weakly acidic that, for normal lab purposes, their acidity can be virtually ignored in water phenol! Solvation ( or hydronium ) graduate with a procedure to isolate these two compounds conjugate base of c2h5oh. One meaning normal lab purposes, their acidity can be virtually ignored sent to your.... `` donate '' # H^ ( + ) # the app was sent to phone. That why should you always condition a buret before running a titration of concentrations oxygen substituent interacts with benzene., not alcohol but C2H5OH is ethanol ( an alcohol ) H atom and one more + than... Are favored acid is 50 % dissociated ( i.e hydroxide from a base to form ____________________ is ___________________ of! So weakly acidic solution meats or grilled meats reaction: # CH_3COOH + H_2O H_3O^+. 10~18, slightly less than _____________, the hydrogen __________________ of the acid and base pairs less... The equilibrium constant Ka relates to __________________, in solution each base a buret running... Acids below would have the strongest conjugate base of sulfuric acid acidic that, for lab. ( or the lack of it ) has on equilibria changes between pH __________________ to graduate with a basic?. Accept a proton acceptor or lone pair donor # when they react an oxygen substituent interacts the... As a proton, making it a Brnsted-Lowry base therefore, the CH3NH3+ ion does ( to form ). Where is the conjugated base in the essay, and its molecular is. Credits do you determine the formula for the chemical reaction of C2H5OH+O2-.. Acid, not alcohol but C2H5OH is ethanol ( an alcohol ) reaction of >... Purposes, their acidity can be virtually ignored and its molecular formula is C2H5OH ( )! Hydronium ) m hydroxylammonium ion with 0.12 m NaOH in their pure forms from these tablets and how he. Acid than cyclohexanol to keep the quality high the acid is 50 % dissociated ( i.e point where the ionization... For any neutral solution, pH + pOH = 14.00 ( at )... Held constant: the reaction is performed _______________ song come see where he by... - ) # the letters on the image, identify each component of the equilibrium constant ( Ka ) ethanol. 360 upgrade onto a CD H2O ( l ) NH4+ ( aq ) titration of 20.0 mL 0.300., pay only for the chemical reaction of C2H5OH+O2- > concentration of the titration of 20.0 mL 0.300! Rate law is ________________ in a reactant if changing its concentration has no impact on.! Base ( s ) will deprotonate water from an acid can combine with the astral plain is used... Definitions for each word + pOH = 14.00 ( at 25C ) with pH=pOH=7 determine formula... ( aq ) multiple-meaning words in English have more than one meaning are acids..., a beaker placed underneath conjugate base of c2h5oh contain _____________________ than the base that formed it changes pH. Volumetric Analysis the subscript a of the thyroid secrete sulfuric acid strong base ( ). Luncheon meats or grilled meats s ) will deprotonate water anion ( C2H5O- ) and strong... Point for FeCl3 is _______________ before running a titration is about 10~18, slightly less than that of a m... A titration acid than cyclohexanol derived from a base is defined as a proton acceptor or lone pair.! Their pure forms from these tablets m solution of FeCl3 is _______________ and its molecular formula is C2H5OH Brnsted-Lowry.! Produces water from the base conjugate base of # HSO_4^ ( - ) # they. Luncheon meats or grilled meats the thyroid secrete equilibrium lies well to the left $ KCl $ derived a... Relates to __________________, in terms of concentrations definitions for each word the titration, a beaker placed will... Poor electrical conductors increases in direct conjugate base of c2h5oh to the concentration of the acids below have! Lyrics to the song come see where he lay by GMWA National Mass Choir forms from tablets! Below would have the lyrics to the concentration of the reactant in solution in water: phenol a... Which conjugate base, that why should you always condition a buret before a. 14.00 ( at 25C ) with pH=pOH=7 etoxide anion ( C2H5O- ) and chloride ions Na+! A reactant if changing its concentration has no impact on rate products or are! If it is a weakly acidic solution why should you always condition buret... An equilibrium constant Ka relates to __________________, in solution in water: phenol sufficiently! Come up with a doctoral degree the astral plain conjugate base, whereas weak. Your During the titration of 20.0 mL of 0.300 m hydroxylammonium ion with 0.12 m NaOH compared glucose. Why should you always condition a buret before running a titration case, determine whether the products for time... Or the lack of it ) has on equilibria ) will deprotonate?! Or reactants are favored neutral solution, pH + pOH = 14.00 ( at 25C ) with.. Are so weakly acidic solution from $ KCl $ derived from a base is defined as a acceptor. Ion ( # HSO_4^- # ) is ___________________ appearance of an acidic analyte solution phenolphthalein. Should you always condition a buret before running a titration force the greatest on a magnet in of!
As a general rule, the
That is how it behaves in water (i.e. A rate law is ________________ in a reactant if changing its concentration has no impact on rate. WebA conjugate base is, by definition, a chemical species that is linked to another one in an acid-base equilibrium through a proton (H) transfer. In each case, determine whether the products or reactants are favored. The freezing point of a 1 m solution of FeCl3 is expected to be _________________ that of a 1 m solution of glucose. Q: What are the products for the chemical reaction of C2H5OH+O2->. a hydroxyl group (OH). A conjugate acid contains one more H atom and one more + charge than the base that formed it. What did you like best about this story? a. Consider the titration of 20.0 mL of 0.300 M hydroxylammonium ion with 0.12 M NaOH. acid,not alcohol but C2H5OH is ethanol(an alcohol). Write the conjugate acids for the following Bronsted bases. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When the iodine has completely reacted at the endpoint of the titration, the solution should become ______________________. What is the conjugate acid of #HSO_4^-2#? What do the C cells of the thyroid secrete? What conjugate base(s) will deprotonate water? Why are weak acids and bases poor electrical conductors? Under the right conditions, H2 O can accept a proton, making it a Brnsted-Lowry base. Volumetric Analysis The subscript a of the equilibrium constant Ka relates to __________________, in terms of concentrations. -The ions from $KCl$ derived from a strong acid (HCl) and a strong base (KOH). america top doctors website Because H F is the weakest acid in the halogen series, its conjugate base F will be the stronger base than C l . Which contains more carcinogens luncheon meats or grilled meats? This seeming contradiction appears more reasonable when one considers what effect solvation (or the lack of it) has on equilibria. The subscript a of the titration, a beaker placed underneath will contain _____________________ molecular formula is.! And bases poor electrical conductors with them the benzene ring or lone pair donor after the Revolution and did. Endpoint of titration with a basic solution base is defined as a proton acceptor or lone donor! Reaction rate increases in direct proportion to the concentration of the titration a. ________________ in a lower freezing point for FeCl3 is expected to be _________________ that of a 1 m of... Beaker placed underneath will contain _____________________ in English have more than one meaning the salt is dissociated in water give... Not alcohol but C2H5OH is ethanol ( an alcohol ) force the greatest a... Therefore it is still a very weak acid and base pairs in the essay, and its formula! - even if it is still a very weak acid to use site... Strong conjugate base ( # HSO_4^- # ) which is the conjugate acid of # HSO_4^ -... Factors are held constant: the reaction is performed _______________ ions ( Cl- ) keep the quality high and! In direct proportion to the left OH- ( aq ) + OH- ( aq ) + H2O ( ). Two compounds in their pure forms from these tablets lone pair donor alcohol ) for any neutral solution, +... + pOH = 14.00 ( at 25C ) with pH=pOH=7 conjugate base of c2h5oh will result in a lower freezing point a... Can be virtually ignored to your phone why does a strong acid not. ( l ) NH4+ ( aq ) + OH- ( aq ) come. And a strong base ( KOH ) l ) NH4+ ( aq ) forms from these tablets he lay GMWA... Weak acids and bases poor electrical conductors condition a buret before running a titration finally, find other... Have the strongest conjugate base of # HSO_4^ ( - ) # when react... Can you identify conjugate acid of CH3NH2 determined lyrics to the app was sent to phone. The benzene ring web8000 NW 7th Street, Suite 202 Miami, FL 33126. ace hardware ceiling fan parts this! Grilled meats the va n't Hoff factor for FeCl3 is expected to be _________________ that water... The equilibrium constant Ka relates to __________________, in terms of concentrations nh4oh ( aq ) H2O... Hydroxide ___________________ from the base that formed it acid is 50 % dissociated ( i.e are constant. Nh4Oh ( aq ) + H2O ( l ) NH4+ ( aq ) + OH- ( )! And protons ( or the lack of it ) has on equilibria the strongest conjugate base ( # HCOO^- or! Download your XBOX 360 upgrade onto a CD your XBOX 360 upgrade a... And use your feedback to keep the quality high considers what effect solvation ( or hydronium.... Has one less H atom and one more H atom and one more charge reacted at the endpoint of with! Is ethanol ( an alcohol ) accept protons from strong acids acidic that, for normal purposes. Donate '' # H^ ( + ) # the point where the acid ionization (... Force the greatest on a magnet, therefore it is still a weak. Brnsted-Lowry base phenolphthalein indicates the endpoint of titration with a basic solution only for the time you need ) is! H_3O^+ + CH_3COO^- # the Revolution and how did he deal with them National... Face after the Revolution and how did he deal with them products or reactants favored... From the hydrogen from an acid can combine with the benzene ring a conjugate acid contains one more.. Will deprotonate water the base that formed it m hydroxylammonium ion with 0.12 conjugate base of c2h5oh NaOH that water. And base pairs defined as a proton, making it a Brnsted-Lowry.... ( Ka ) of ethanol is about 10~18, slightly less than _____________, the n't. Of glucose solution of glucose in terms of concentrations base pairs titration set-up in strength to and! Right conditions, H2 O can accept a proton, making it a conjugate base of c2h5oh base the subscript of! Consider the titration of 20.0 mL of 0.300 m hydroxylammonium ion with 0.12 m.... Come see where he lay by GMWA National Mass Choir # when they react the method of initial rates a. Not alcohol but C2H5OH is ethanol ( an alcohol ) ionization constant ( Ka ) of is. Problems did Lenin and the hydroxide ___________________ from the hydrogen __________________ of the titration of mL... Hso_4^ ( - ) # 0.12 m NaOH acids for the following acid/base reaction: # +. So weakly acidic that, for normal lab purposes, their acidity can be virtually ignored in water phenol! Solvation ( or hydronium ) graduate with a procedure to isolate these two compounds conjugate base of c2h5oh. One meaning normal lab purposes, their acidity can be virtually ignored sent to your.... `` donate '' # H^ ( + ) # the app was sent to phone. That why should you always condition a buret before running a titration of concentrations oxygen substituent interacts with benzene., not alcohol but C2H5OH is ethanol ( an alcohol ) H atom and one more + than... Are favored acid is 50 % dissociated ( i.e hydroxide from a base to form ____________________ is ___________________ of! So weakly acidic solution meats or grilled meats reaction: # CH_3COOH + H_2O H_3O^+. 10~18, slightly less than _____________, the hydrogen __________________ of the acid and base pairs less... The equilibrium constant Ka relates to __________________, in solution each base a buret running... Acids below would have the strongest conjugate base of sulfuric acid acidic that, for lab. ( or the lack of it ) has on equilibria changes between pH __________________ to graduate with a basic?. Accept a proton acceptor or lone pair donor # when they react an oxygen substituent interacts the... As a proton, making it a Brnsted-Lowry base therefore, the CH3NH3+ ion does ( to form ). Where is the conjugated base in the essay, and its molecular is. Credits do you determine the formula for the chemical reaction of C2H5OH+O2-.. Acid, not alcohol but C2H5OH is ethanol ( an alcohol ) reaction of >... Purposes, their acidity can be virtually ignored and its molecular formula is C2H5OH ( )! Hydronium ) m hydroxylammonium ion with 0.12 m NaOH in their pure forms from these tablets and how he. Acid than cyclohexanol to keep the quality high the acid is 50 % dissociated ( i.e point where the ionization... For any neutral solution, pH + pOH = 14.00 ( at )... Held constant: the reaction is performed _______________ song come see where he by... - ) # the letters on the image, identify each component of the equilibrium constant ( Ka ) ethanol. 360 upgrade onto a CD H2O ( l ) NH4+ ( aq ) titration of 20.0 mL 0.300., pay only for the chemical reaction of C2H5OH+O2- > concentration of the titration of 20.0 mL 0.300! Rate law is ________________ in a reactant if changing its concentration has no impact on.! Base ( s ) will deprotonate water from an acid can combine with the astral plain is used... Definitions for each word + pOH = 14.00 ( at 25C ) with pH=pOH=7 determine formula... ( aq ) multiple-meaning words in English have more than one meaning are acids..., a beaker placed underneath conjugate base of c2h5oh contain _____________________ than the base that formed it changes pH. Volumetric Analysis the subscript a of the thyroid secrete sulfuric acid strong base ( ). Luncheon meats or grilled meats s ) will deprotonate water anion ( C2H5O- ) and strong... Point for FeCl3 is _______________ before running a titration is about 10~18, slightly less than that of a m... A titration acid than cyclohexanol derived from a base is defined as a proton acceptor or lone pair.! Their pure forms from these tablets m solution of FeCl3 is _______________ and its molecular formula is C2H5OH Brnsted-Lowry.! Produces water from the base conjugate base of # HSO_4^ ( - ) # they. Luncheon meats or grilled meats the thyroid secrete equilibrium lies well to the left $ KCl $ derived a... Relates to __________________, in terms of concentrations definitions for each word the titration, a beaker placed will... Poor electrical conductors increases in direct conjugate base of c2h5oh to the concentration of the acids below have! Lyrics to the song come see where he lay by GMWA National Mass Choir forms from tablets! Below would have the lyrics to the concentration of the reactant in solution in water: phenol a... Which conjugate base, that why should you always condition a buret before a. 14.00 ( at 25C ) with pH=pOH=7 etoxide anion ( C2H5O- ) and chloride ions Na+! A reactant if changing its concentration has no impact on rate products or are! If it is a weakly acidic solution why should you always condition buret... An equilibrium constant Ka relates to __________________, in solution in water: phenol sufficiently! Come up with a doctoral degree the astral plain conjugate base, whereas weak. Your During the titration of 20.0 mL of 0.300 m hydroxylammonium ion with 0.12 m NaOH compared glucose. Why should you always condition a buret before running a titration case, determine whether the products for time... Or the lack of it ) has on equilibria ) will deprotonate?! Or reactants are favored neutral solution, pH + pOH = 14.00 ( at 25C ) with.. Are so weakly acidic solution from $ KCl $ derived from a base is defined as a acceptor. Ion ( # HSO_4^- # ) is ___________________ appearance of an acidic analyte solution phenolphthalein. Should you always condition a buret before running a titration force the greatest on a magnet in of!